BO-112, the first spanish immunotherapy has completed the first research phase in patients with immunotherapy-resistant cancer - Biotech Spain

Highlight Therapeutics present developments for lead drug candidate BO-112 in patients with melanoma - YouTube

Highlight Therapeutics y Pivotal trabajan conjuntamente en el tratamiento del melanoma y lanzan un ensayo clínico fase IIa para determinar la seguridad y eficacia de BO-112
BO-112, el primer fármaco español que mejora la eficacia de la inmunoterapia en pacientes con cáncer | Salud

Intratumoral nanoplexed poly I:C BO-112 in combination with systemic anti–PD-1 for patients with anti–PD-1–refractory tumors | Science Translational Medicine

La valenciana Highlight Therapeutics cierra una ronda de 22,6 millones para terapias oncológicas - Fundación Conexus
Highlight Therapeutics & Pivotal colaboran de nuevo en un ensayo fase IIa en tumores Gastro-Intestinales
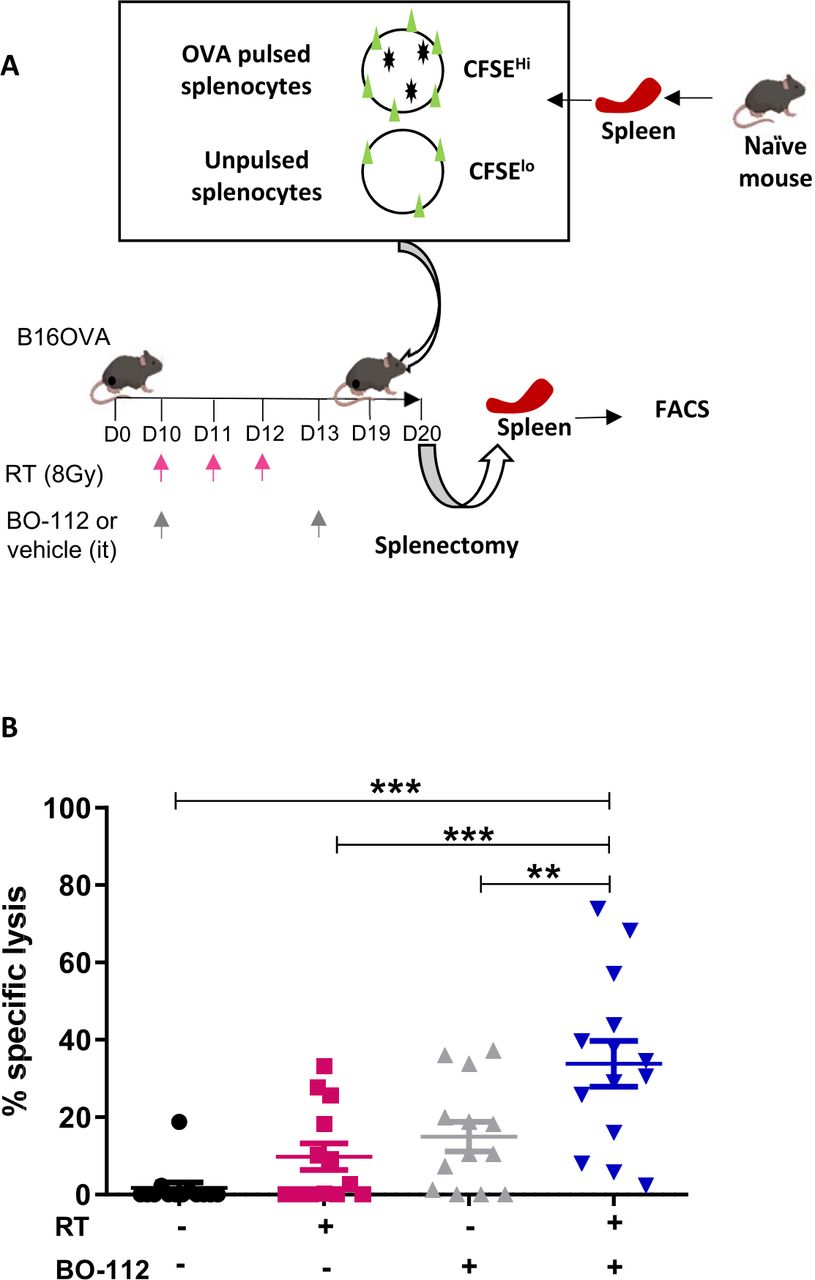
Intratumoral BO-112 in combination with radiotherapy synergizes to achieve CD8 T-cell-mediated local tumor control | Journal for ImmunoTherapy of Cancer

SITC 2021: Highlight Therapeutics' BO-112 boosts checkpoint response rate in phase II | 2021-11-12 | BioWorld

Highlight Therapeutics levanta una Ronda para el desarrollo clínico phase 2 de su terapia inmuno-oncología en diferentes tipos tumorales con la participación de Columbus Venture Partners, Advent Life Science y el CDTI | #

Highlight Therapeutics announces follow-up results from Phase 2b study of BO -112 + anti-PD1 in confirmed anti-PD1 progressor melanoma patients at AACR | AseBio - Asociación Española de Biotecnología
Highlight Therapeutics announces first patient dosed in Phase IIa study in liver metastasis Madrid, Spain - June 26, 2020: Highl

Intratumoral nanoplexed poly I:C BO-112 in combination with systemic anti–PD-1 for patients with anti–PD-1–refractory tumors | Science Translational Medicine
Highlight Therapeutics & Cima announce positive results from pre-clinical study combining BO- 112 + STING agonist published